Lipids
Lipids refer to a group of molecules comprising fats, oils, phospholipids, waxes and steroids. All lipids are hydrophobic and don’t dissolve in water. However, they dissolve in organic solvents. The backbone of all lipid compounds is Glycerol or Glycerine. Glycerol is a sugar alcohol, made of a linear chain of three carbon atoms and three hydroxyl groups. It is soluble in water.
Hydrophobic and Hydrophilic molecules
Hydrophobic molecules are molecules which don’t dissolve in water (hydro = water, phobia = fear). Hydrophilic molecules dissolve in water (philia = friendship). Water is a polar substance. The thumb rule is that “equal dissolves equal”, so, hydrophobic substances are non-polar molecules whereas hydrophilic molecules are polar molecules. Fats and oils are hydrophobic molecules, meaning that they are non-polar and insoluble in water. Lipids in general are molecules with a large non-polar extension, making them soluble in non-polar solvents, such as benzene, ether and chloroform. There exist some amphipathic lipids (example Phospholipids) which are soluble in water as well as organic solvents.
Fats and Oils
The fats are triglycerides made of three molecules of fatty acids bound to one molecule of glycerol. Thus, fats are also known as triesters of glycerol. Fats are not soluble in water but soluble in organic solvents.
Phospholipids
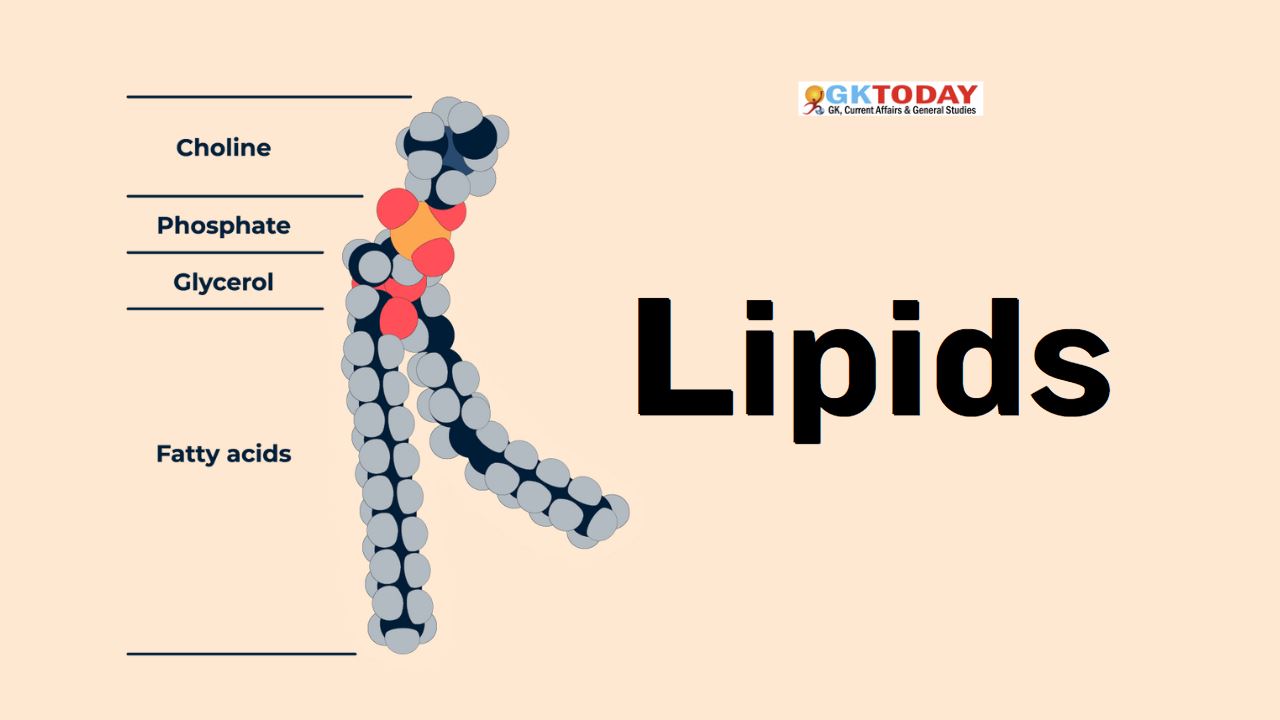
Phospholipids are molecules made up of one molecule of glycerol bound to two molecules of fatty acids and also one phosphate group. They are main components of the cell membranes. Phospholipids are amphipathic molecules, meaning that they have a non-polar portion, due to the long fatty acid chains, and a polar portion, due to the phosphate group. They dissolve in water as well as organic solvents.
Steroids
Steroids are another class of lipids, which have a unique chemical structure. They are built from four carbon-laden fused ring structures. Bile salts, cholesterol, the sexual hormones estrogen, progesterone and testosterone, corticosteroids and pro-vitamin D are examples of steroids. Their functions are as follows:
- Aldosterone : Maintains water and salt balance by the kidney, controls blood pressure
- Bile acids : Produced by the liver, help in the digestion of dietary lipids
- Cholesterol : Provides stability and flexibility to cell membranes
- Cortisone : Carbohydrate metabolism
- HDL (high density lipoproteins) and LDL (low density lipoproteins): Lipid-protein combinations that transport lipids in the blood
- Testosterone, estrogens, progesterone: Maintain sex characteristics. Allow reproduction to occur.
Saturated and Unsaturated Fats
In Saturated fats, the Carbon molecule is bound to as many hydrogen molecules as many it is possible. Thus, all C-C bonds in saturated fats are single bonds only. There are no double or triple bonds in saturated fats. Generally, saturated fats are solid at room temperature. Examples of saturated fat are ghee, cream, cheese, butter etc.
In unsaturated fats, double and triple C-C bonds are found, and thus there is a possibility of adding few more hydrogen atoms. Generally, unsaturated facts are liquid at room temperature. If there are more than carbon-carbon double / triple bonds present, such fat is called Poly Unsaturated Fatty Acid (PUFA). Examples of such PUFA include palmitoleic acid, oleic acid, myristoleic acid, linoleic acid, and arachidonic acid.
Hydrogenation: Converting Unsaturated Fat to Saturated Fat
The unsaturated fatty acids have double bonds, and therefore have fewer hydrogen atoms than maximum possible. The process of hydrogenation can convert an unsaturated fat into saturated fat by adding extra hydrogen atoms to it. Thus, hydrogenation converts liquid vegetable oils into solid or semi-solid fats. This reaction is the basis of Vegetable Oil industry and is achieved in the presence of some catalysts such as nickel, palladium or platinum metals. This method has prevented oxidation and thus rancidity and has allowed for the development of foods with less animal and saturated fats. However, the consumption of hydrogenated fatty acids increases risk of heart disease, because the fats cause a change in the structure of targeted unsaturated fatty acids. Kindly note that majority but not all double / triple bonds broken during hydrogenation of unsaturated fats. Hydrogenation may also result in creation of unsaturated fats with peculiar hydrogen atoms arrangement called “Trans Fats”.
Trans and Cis Fats
Cis and trans are terms that refer to the arrangement of the two hydrogen atoms bonded to the carbon atoms involved in a double bond in unsaturated fats. There are no cis or trans types in saturated fats because they have single bonds only.
In the cis arrangement, the hydrogen atoms are on the same side of the double bond. In the trans arrangement, the hydrogens are on opposite sides of the double bond.

We note here that most naturally occurring fats are Cis fats. Only a handful of naturally occurring fats are trans fats such as those found in milk and body fat of ruminants (such as cattle and sheep). Further, trans fats are generated during hydrogenation processing of polyunsaturated fatty acids in food production. They are outcome of the Partial Hydrogenation and not the complete Hydrogenation, because complete Hydrogenation would end the double bonds.
The process of hydrogenation adds hydrogen atoms to unsaturated fats, eliminating double bonds and making them into partially or completely saturated fats. However, partial hydrogenation, if it is chemical rather than enzymatic, converts a part of cis-isomers into trans-unsaturated fats instead of hydrogenating them completely.
Impacts of Trans fats on health
The consumption of trans fats has been shown to slightly increase the levels of bad cholesterol (LDL) in the blood. However, as per recommendations of the US National Academy of Sciences (NAS), trans fats are not essential and provide no known benefit to human health”, whether of animal or plant origin. While both saturated and trans fats increase bad cholesterol; the trans unsaturated fats also lower levels of good cholesterol. In this way, trans fats increase the risk of heart diseases.
Good Fats or Bad Fats
One thing is clear that no fats are “bad,” as fats are excellent sources of energy and help to maintain the health of the body. However, Fat is only bad if it is too much. There are several fats that are considered essential (the omega-6 and omega-3 fatty acids)-in other words, they are substances that our bodies require for maintenance but that we cannot manufacture. These are considered to be “good” fats. Comparatively, the fats we don’t need to ingest are often dubbed as “bad.”
Thermal Properties of Fats and Lipids
Fats are poor heat conductors and they also form thick layers of fatty tissue (called adipose tissue) when accumulated in an organism. This is the reason that they serve as good thermal insulators. In cold climate fauna such as polar bears, seals and whales, adipose tissue helps the maintenance of internal body temperature.
Fats as source of Energy
In carbohydrates are the main energy sources for aerobic cell respiration. However, when carbohydrates are absent or deficient, the body can use lipid (and also proteins) to break them and get energy.