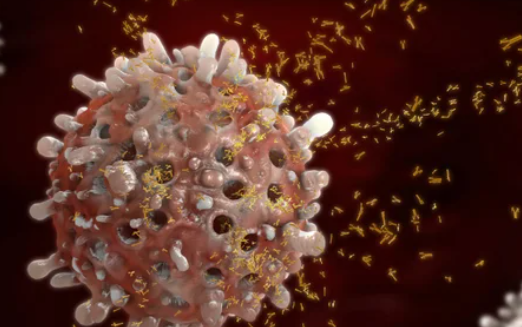
What is Radical CAR-T Therapy?
Car-T therapy, which is also called “chimeric antigen receptor T-cell” therapy, is a new and innovative way to treat cancer. Recently, in November 2023, the US Food and Drug Administration (FDA) began looking into whether Car-T therapy could cause new types of cancer. By March 2024, the FDA had found 33 cases of secondary cancers in about 30,000 patients who had been treated. This led to all Car-T treatments having to have a boxed warning.
Understanding Car-T Therapy
Car-T treatment takes T-cells from a patient, changes them in a lab so they can better fight cancer cells, and then adds more of them to the patient’s body. This medicine has worked very well, especially for lymphomas and multiple myeloma which are hard to treat.
Potential Causes of Secondary Cancers
The genetic changes that happen during Car-T treatment may be linked to the secondary cancers that were seen. Retroviruses used in the process could lead to “insertional mutagenesis,” a process in which new genetic material could change how genes normally work and possibly cause cancer. But it’s still not clear if these new cases of cancer are directly caused by Car-T treatment.
Relative Risks and Benefits
There is a chance of getting a second cancer, but it doesn’t happen very often. The medicine has been very important for people with advanced cancer, where the benefits are much greater than the risks. Also, Car-T therapy is being used more and more in other areas, like treating autoimmune diseases and stopping organ graft rejection.
Ongoing Research and Future Directions
More research is being done to make the viral vectors used in Car-T therapy safer and to see if they could be used to treat other serious diseases. Medical professionals are also dedicated to telling patients about the rare but possible risks of Car-T therapy so that patients can make smart choices about their treatment options.
About Car-T therapy
- Revolutionary Treatment: CAR-T therapy, or Chimeric Antigen Receptor T-cell therapy, revolutionizes cancer treatment by genetically engineering patients’ T-cells to target cancer cells. Approved by the FDA in 2017, it is primarily used against acute lymphoblastic leukemia and certain non-Hodgkin lymphomas.
- Living Drug Approach: This one-of-a-kind “living drug” method changes a patient’s T-cells outside of the body and then puts them back in to fight cancer cells.
- Challenges and Research: Even though CAR-T treatment works very well, it can cause neurotoxicity and cytokine release syndrome that are very bad. Researchers are still working on ways to make it safer and cheaper so that it can be used on large tumors as well.
Month: Current Affairs - July, 2024
Category: Science & Technology Current Affairs